Smart dust is about to enter our lives1,2. Computers, sensors and robots as small as a grain of salt are being developed that can move around, detect light, sound, pressure, chemicals and magnetic fields. Less than a millimetre across and a few hundred micrometres thick, they process information and communicate wirelessly. They have a range of uses from medical diagnostics, surgery and brain monitoring to tracking butterflies and the conditions of crops.
But how to power them? The smallest battery is around 2 square millimetres in area3, or several times larger than a smart dust chip. And it’s not powerful enough to continuously drive the complex functions of a device. Smart dust chips thus rely on external sources of power, such as solar panels. However, these don’t work at night or on a foggy day.
Batteries need to be shrunk. But it’s hard to squeeze all the components into a tighter space. They also need to be built into microdevices, not just bolted on. It’s the equivalent, on a much smaller scale, of Tesla’s efforts to make electric-vehicle batteries an integral part of its cars. At whatever scale, the techniques used to make batteries and electronic devices are different.
Compact batteries, such as lithium-ion ones, are produced using wet chemistry — painting slurries of materials on metal foils, for instance. Tweaking the materials’ composition can improve performance only by so much.
By contrast, microelectronics engineers sculpt semiconductor wafers to perform certain functions, using methods such as etching and deposition. But these don’t work well with battery materials. Fundamentally different designs are needed.
Tiny batteries need advances in both areas: energy-dense, durable materials to improve charge storage; and clever architectures to shrink and combine components.
As nano-scientists who build tiny devices, we’ve seen first-hand how hard it is to combine electrochemistry and microelectronics. These disciplines have developed separately. Micro-electronics engineers struggle to accommodate new materials, such as active polymers, into their processes. Cross-contamination and mismatched thermal and electronic properties are common problems. Battery and materials scientists, meanwhile, are often satisfied with optimizing a material against one parameter, without considering its use in devices and circuits. That’s why we built an interdisciplinary team spanning these fields in our laboratory.
Here, we call on electrical engineers, battery and polymer scientists to work more closely to overcome these problems. We highlight research priorities for redesigning battery structures, materials and fabrication methods. We also call on funders and universities to train more scientists with the cross-disciplinary research skills needed to build the next generation of microtechnologies.
Four ways
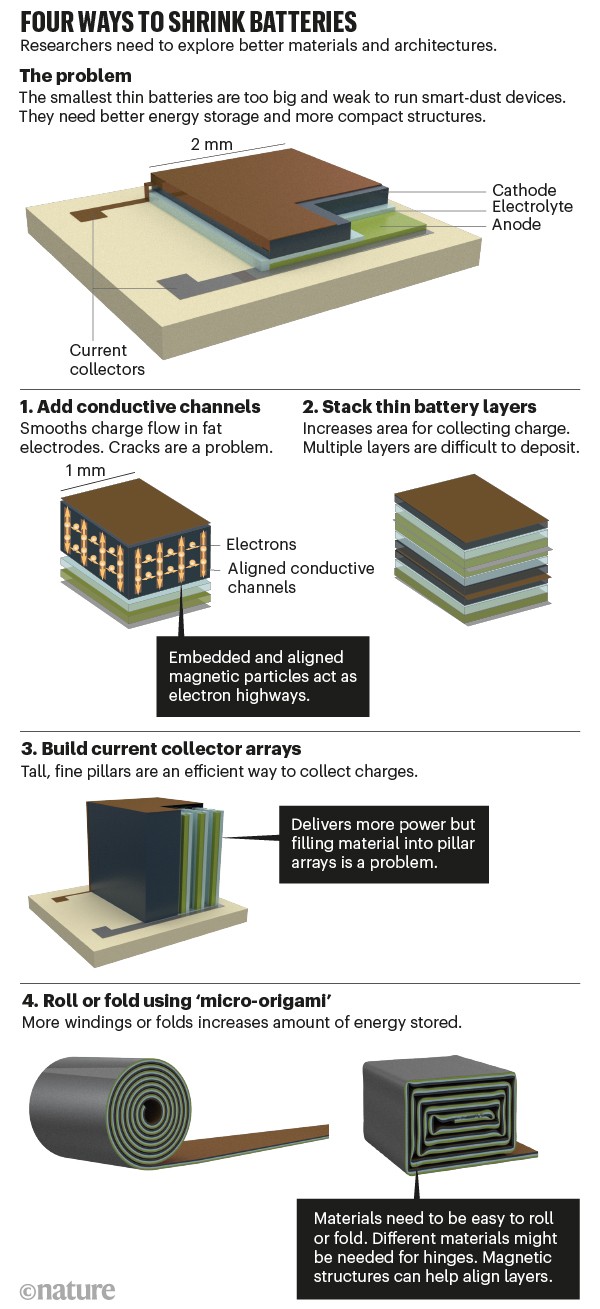
A battery is essentially a sandwich of many layers. Two electrodes store electrical power as chemical energy. In between, an electrolyte mediates the flow of charges without short circuiting. Two metal current collectors connected to the electrodes direct power to external circuits. However, the smaller the electrodes are, the less charge they can hold. Cracks and other defects might stop the flow of electrons and cause the battery to fail. And tortured passages of ions and electrons in fat material layers increase resistance.
To bypass some of these problems, the smallest batteries are very thin. But they don’t pack much of a punch. Their energy density per unit area is low, around 800 times less than a centimetre-sized lithium-ion coin cell. A thin-film battery 2 mm2 in area and 150 micrometres thick can power a simple temperature sensor for 2 days. But it can’t deliver enough to transmit data for an hour3.
Here are four ways to store more charge in a smaller space (see ‘Four ways to shrink batteries’).
One, add conductive channels to thick electrodes. Like painted lanes on a motorway, embedded rows of magnetic particles keep charges moving smoothly4. However, such methods haven’t yet been demonstrated on millimetre scales. It’s difficult to lay down chains of particles accurately. Cracks remain a problem.
Two, stack many thin battery-sandwiches together. This keeps charges flowing cleanly. But it’s hard to deposit many layers reliably, let alone keep them aligned. For example, the high temperature needed to anneal one electrode layer might destroy others below. Some materials don’t sit well on top of others. And mismatches grow as the stack builds. Defects might cause short circuits between close-spaced electrodes.
Three, re-engineer the current collectors. Building them in as pillars rather than sheets, and thus making the structure 3D, increases the contact area with electrodes and electrolytes, and therefore the efficiency of drawing power. It is feasible to build such fine structures in 3D by etching them into a silicon wafer, for example. But it is extremely fiddly to include additional steps, such as coating electrode materials, to assemble the whole device. It’s not been achieved yet on microscales.
Four, fold or roll films using ‘micro-origami’. At larger scales, this can be done by hand5; in commercial block or cylinder batteries, a folding or winding machine is used. At millimetre scales, self-assembly is another way to do it. Thin films can be made to roll themselves by building in and releasing tension. Our group has done this with tiny capacitors, which are sheets of dielectrics sandwiched between metals6. But, like rolling up a poster, it’s hard to wind films hundreds of times without them becoming misaligned. Magnetic guiding can help: incorporating a tiny amount of ferromagnetic material into the battery film and applying a magnetic field can keep the curling procedure on track. Although we’ve shown this with capacitors, battery stacks are much harder to deal with. They are thicker and their mechanical behaviour is harder to predict.
Folding is even more challenging. It takes increasing amounts of force to bend the growing stack, as with doubling over a piece of paper several times. Hinges accumulate stress and crack. A ‘self-folding’ process would need to take all these details into account, for example by incorporating different materials into hinges. But, it would still be hard to align all the layers and components.
Folding a thin-film battery 30 times into an area that fits into the smallest computer (0.14 mm2) could, we estimate, power it for at least 100 days with one charge1,7. Many smart-dust applications will need more-powerful batteries with hundreds of folds.
Improve materials
Microbatteries also need advances in materials, so that films can be made as thin as possible, to aid the micro-origami and enhance charge storage. Lithium-ion and aqueous zinc batteries are the most developed chemistries. The challenge is fabricating them in ways that are compatible with semiconductor technologies.
In lithium-ion batteries, cathode materials (usually metal oxides such as LiMn2O4 and LiCoO2) are workable on tiny scales, by etching or lifting off redundant materials. The anodes (typically graphite) and electrolytes are harder to handle, with electrolytes often made of liquid organic compounds soaked into a matrix or separator. Solid electrolytes can be fashioned. But ceramics lose their conductivity when very thin, and are brittle. Polymers can be shaped, but the processes for doing so (such as ion etching and photo curing) must be fine-tuned — by building links into their molecular chains that are easy to form or break, for example. Other methods need to be honed, such as spin coating or depositing polymer electrolytes in gas phase. The conductivity of polymer electrolytes also needs improving, to be competitive with liquid ones.
Anodes are needed that hold more charge. Silicon and lithium anodes are being explored. But they need to be stabilized. Silicon reacts with lithium and swells as the battery charges, eventually pulverizing the electrode. Nanotechnology could avoid such damage, for example by wrapping silicon in graphene nano-sheets and using polymers to accommodate the volume change. These solutions must also be adapted to work with on-chip fabrication.
Anodes made from a sliver of metallic lithium also have short life cycles. Lithium is stripped off as the battery runs and rebuilds after charging. But the replacement process is imperfect and the anode gradually wears away over hundreds of cycles. Lithium needs to be better managed in the microfabrication process. One way is to avoid using a sliver of metal and effectively build a lithium electrode from ions still in the electrolyte during charging. Such a battery on a 5-mm2 chip can cycle 80 times7. Still, that’s way off the lifetimes of 5–25 years needed for implantable medical devices.
Aqueous zinc batteries also need better electrodes. Zinc, used as the anode, is efficient at storing and discharging ions. Acidic electrolytes can be better than the typical alkaline ones. But the zinc dissolves in acid and releases hydrogen. So the anode has to be protected with an anti-corrosion layer, or the electrolyte needs to be modified to release fewer protons. Similarly, the cathode (generally made from metal oxides such as MnO2 and V2O5) is susceptible to acid and needs a barrier layer.
Such batteries also need to work at higher voltages — at more than about 2V, a water-splitting reaction takes place. This problem needs to be overcome because the reaction consumes energy. Pathways need to be explored for all the intermediary ions involved in carrying charge (including H+, Zn2+, Mn2+ and OH−) and their interactions with electrode materials8. Polymer-based electrolytes might offer a buffer against water-splitting.
Other battery chemistries are emerging, such as ones that use Mg, Ca, K, and Na ions. These are not yet mature enough for making microbatteries.
Next steps
Materials and microelectronics researchers need to learn from each other. It is so frustrating when a material works well in a lab but little of that performance can be rescued in a real device. We must go to one another’s lab benches, spend a few days designing and making each other’s prototypes and understanding each other’s challenges. For example, how can the polymer electrolyte withstand the wet chemistry needed to pattern metal layers above it? New processes will be needed to synthesize materials on wafer chips at a given position and layer in the battery stack.
Materials conferences, such as those of the US-based Materials Research Society, the American Chemical Society and the American Physical Society, should invite electronics engineers to attend sessions on energy storage. Electronics conferences, such as the VLSI International Symposia on semiconductor technologies, should invite material scientists to share their state-of-the-art know-how on battery chemistries. One goal is to develop a joint roadmap for microbattery performance and target specifications.
Computer modelling will be essential, aided by machine-learning algorithms9. Optimizing construction and materials is experimentally demanding. Any change of material (crystallinity, thickness and synthesis routes) alters the mechanics, stability and therefore the origami behaviour of the films. Tedious work needs to be done to optimize each parameter, such as strain or battery chemistry. Designers need to understand how electrochemical and mechanical properties influence the self-assembly process.
Plans are needed to generate and share reproducible data for batteries and microdevices. The Joint Center for Energy Storage Research in Illinois and the European Battery 2030+ initiative foster collaboration towards a next-generation battery, including smart functions, durable materials and industrial manufacturing.
Universities need to offer cross-disciplinary courses in materials chemistry and micro-electronic technology. Funding should come from both fields. China is moving fast in this direction. In August, China’s Ministry of Education set up an interdisciplinary subject combining electronics, engineering, materials, chemistry and physics, putting it on a par with pure disciplines such as natural sciences. More than US$2 billion has been invested to establish a new campus at the Hong Kong University of Science Technology, a world-leading research institution, in Guangzhou, China. It will follow a hub model. For example, the Function Hub will blend materials and microelectronics knowledge to improve the integration of micro- and nano-devices into multifunctional components. At the Chemnitz University of Technology in Germany, one of us (O.G.S.) teaches a similar course called materials in micro- and nanotechnologies. It mixes photonics, electronics, biotechnology, microrobotics and energy storage to prepare students for the complex microsystems engineering of the future.
With such coordinated efforts, microbatteries will pave the way for imperceptible and pervasive computing within a decade.
"do it" - Google News
January 13, 2021 at 06:48PM
https://ift.tt/35zGYZU
Tiny robots and sensors need tiny batteries — here's how to do it - Nature.com
"do it" - Google News
https://ift.tt/2zLpFrJ
https://ift.tt/3feNbO7
Bagikan Berita Ini














0 Response to "Tiny robots and sensors need tiny batteries — here's how to do it - Nature.com"
Post a Comment